Primary Research Report:
Research on Trout Spawning Cycles
The following reports on a study designed to compare spawning time and egg quality of rainbow trout broodstock maintained in cold creek water with those of broodstock maintained in constant temperature spring water. Included is a discussion of the research background, the methods and facilities used in the present study, and the results and conclusions drawn from the experiment.
Research Background
It is often desirable to provide eggs or fry from a particular strain of rainbow trout (Salmo gairdneri) over an extended period of time. Availability can be prolonged by incubating eggs in cold water as this slows embryonic development. However, an experiment conducted at the Bozeman Fish Cultural Development Center in which rainbow trout eggs were exposed to temperatures of 13, 10, 6, and 3° C (55.5, 50, 43, 37.5° F) showed a significant reduction in the percentage of eyed eggs and the survivability of eggs held at the lowest temperatures (unpublished data). Published studies on egg incubation of various salmonid species indicate that temperatures below 4.5° C (40° F) are detrimental (Davis 1953; Combs and Burrows 1957; Combs 1965; Hokanson et al. 1973; Leitritz and Lewis 1976). Another possible method of extending egg availability is to hold broodstock in cold water, and thus delay egg and sperm development. The present study was designed to compare spawning time and egg quality of rainbow trout broodstock maintained in cold creek water with those of broodstock maintained in constant temperature spring water.
The facilities at the Bozeman Center offer the opportunity to use Bridger Creek or spring water in the raceways. Chemical characteristics of the water supplies are similar. Alkalinity and total hardness (both in mg/L as CaCO3) and pH of the creek water were 189, 203 and 8.2, respectively (Russo and Thurston 1974); values for spring water were 181, 207 and 7.6 (analysis by Water Chemistry Laboratory, Montana State University, Bozeman, Montana).
Methods
Two hundred fifty, 3-year-old, Winthrop strain rainbow trout were held in each of two outdoor raceways measuring 1.8 x 18 0.6 m (6 x 60 x 2 ft). The trout had been reared in spring water and the raceways were initially provided with this water. In early September, creek water was introduced into one raceway; the other continued to receive spring water at 10 " 1° C (50 " 1° F). Water inflow for each raceway was approximately 570 L/min (150 gal/min). Fish were checked weekly beginning in December and artificially spawned by manually stripping when ripe. The initial spawn from each group was discarded; egg quality was believed to be poor because optimum spawning time was missed.
Fish were anesthetized in a 1% salt solution containing 50 mg/l of MS 222 and then checked for ripeness. Ripe females were rinsed with fresh water and the eggs spawned into a strainer to drain off excess fluid. Eggs were then placed in a pan and fertilized. On each spawning day, nine to 18 females were spawned separately and fertilized with sperm from separate males. If additional females were spawned, eggs from three fish were pooled and fertilized with sperm from two males. Eggs were incubated in individual and pooled lots in Heath incubator trays supplied with spring water at the rate of 19 L/min (5 gal/min). Eggs were treated daily with formulin at a concentration of 1:600 for 15 min to control fungus. When eyed, eggs were counted with a Veeder-Root electronic counter. Dead eggs were picked out by hand with a suction bulb and glass tube.
Data collected for individual fish included female weight, total number of eggs per kilogram of body weight, and percent of eyes eggs (Table 1). A comparison of two samples test (t-test) was used to statistically analyze the data (Snedecor and Cochran 1967).
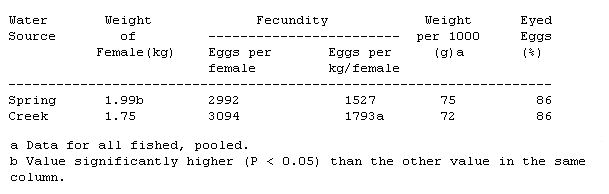
Table 1. Statistics on egg production of rainbow trout broodstock held in spring water at 10° C (50° F) and Bridger Creek at lower temperatures.
Results
Average weekly temperatures of the creek water ranged from 2° C (35° F) to 14° C (57° F) (Figure 1). Fish held in spring water were spawned from December 12, 1977, to February 9, 1978, and fish held in creek water from March 29 to April 20, 1978 (Figure 2). When fish held in creek water were ready to spawn, water temperature was about 7° C (45° F) and rising rapidly. At this time, the creek water became heavily silted and was replaced with spring water.
A total of 39 females were spawned from the creek water and 66 females from the spring water. Gross observations such as "blood in eggs" or "some bad eggs" were made when spawn was taken. Fish observed with these conditions were not considered in the individual comparisons, because these eggs would normally be discarded. However, the data were included when overall egg weight per thousand eyed eggs was calculated (see Table 1).
Fish held in spring water were significantly larger at spawning time than those held in creek water; a difference directly related to increased food intake and higher metabolism at the higher water temperatures. Fish held in creek water produced more eggs per kilogram of body weight than did those in spring water, but the eggs were smaller; consequently, there was no significant difference in weight of eggs per kilogram of fish between groups. Neither was there a significant difference in total number of eggs per female or in percent eyed eggs between the fish held in spring water and those held in creek water (see Table 1).
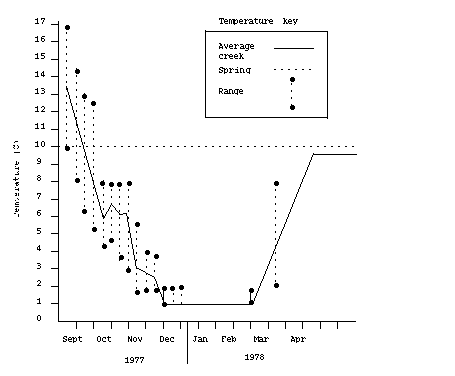
Figure 1. Average temperature and ranges for
creek versus spring water, beginning September 6,1977 (Week 1).
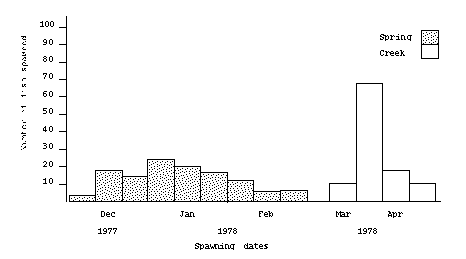
Figure 2. Number of rainbow trout held in spring
water or creek water, spawned on different dates.
Conclusions
This study suggests that, if late-season rainbow trout eggs are desired, broodfish (at least of the Winthrop strain, and perhaps other late fall and winter strains) can be held in cold water to delay spawning without affecting egg quality.
We also demonstrated that a domestic hatchery strain of fish that spawns in early winter in a hatchery environment can revert to typical spring spawning when exposed to temperatures experienced in the wild.
References
Combs, B.D. 1965. Effects of temperature on the development of salmon eggs. Progressive Fish-Culturist 27:134-137.
Combs, B.D., R.E. Burrows. 1957. Threshold temperatures for the normal development of chinook salmon eggs. Progressive Fish-Culturist 19:3-6.
Davis, H.S. 1953. Culture and disease of game fishes. University of California Press, Berkeley.
Hokanson, K.F.R., J.H. McCormick, B.R. Jones, and J.H. Tucker. 1973. Thermal requirements for maturation, spawning, and embryo survival of the brook trout, Salvelinus fontinalis. Journal of the Fisheries Research Board of Canada 30:975-984.
Leifritz, E., and R.C. Lewis. 1976. Trout and salmon culture (hatchery methods). California Department of Fish and Game, Fish Bulletin 164.
Russo, R.C., and R.V. Thurston. 1974. Water analysis of Bridger Creek (Gallatin County) Montana 1973. Montana State University, Fisheries Bioassay Laboratory, Technical Report 74-3, Bozeman.
Snedecor, G.W., and W.G. Cochran. 1967. Statistical methods. Iowa State University Press, Ames.